-
 Afrikaans
Afrikaans -
 Albanian
Albanian -
 Amharic
Amharic -
 Arabic
Arabic -
 Armenian
Armenian -
 Azerbaijani
Azerbaijani -
 Basque
Basque -
 Belarusian
Belarusian -
 Bengali
Bengali -
 Bosnian
Bosnian -
 Bulgarian
Bulgarian -
 Catalan
Catalan -
 Cebuano
Cebuano -
 Corsican
Corsican -
 Croatian
Croatian -
 Czech
Czech -
 Danish
Danish -
 Dutch
Dutch -
 English
English -
 Esperanto
Esperanto -
 Estonian
Estonian -
 Finnish
Finnish -
 French
French -
 Frisian
Frisian -
 Galician
Galician -
 Georgian
Georgian -
 German
German -
 Greek
Greek -
 Gujarati
Gujarati -
 Haitian Creole
Haitian Creole -
 hausa
hausa -
 hawaiian
hawaiian -
 Hebrew
Hebrew -
 Hindi
Hindi -
 Miao
Miao -
 Hungarian
Hungarian -
 Icelandic
Icelandic -
 igbo
igbo -
 Indonesian
Indonesian -
 irish
irish -
 Italian
Italian -
 Japanese
Japanese -
 Javanese
Javanese -
 Kannada
Kannada -
 kazakh
kazakh -
 Khmer
Khmer -
 Rwandese
Rwandese -
 Korean
Korean -
 Kurdish
Kurdish -
 Kyrgyz
Kyrgyz -
 Lao
Lao -
 Latin
Latin -
 Latvian
Latvian -
 Lithuanian
Lithuanian -
 Luxembourgish
Luxembourgish -
 Macedonian
Macedonian -
 Malgashi
Malgashi -
 Malay
Malay -
 Malayalam
Malayalam -
 Maltese
Maltese -
 Maori
Maori -
 Marathi
Marathi -
 Mongolian
Mongolian -
 Myanmar
Myanmar -
 Nepali
Nepali -
 Norwegian
Norwegian -
 Norwegian
Norwegian -
 Occitan
Occitan -
 Pashto
Pashto -
 Persian
Persian -
 Polish
Polish -
 Portuguese
Portuguese -
 Punjabi
Punjabi -
 Romanian
Romanian -
 Russian
Russian -
 Samoan
Samoan -
 Scottish Gaelic
Scottish Gaelic -
 Serbian
Serbian -
 Sesotho
Sesotho -
 Shona
Shona -
 Sindhi
Sindhi -
 Sinhala
Sinhala -
 Slovak
Slovak -
 Slovenian
Slovenian -
 Somali
Somali -
 Spanish
Spanish -
 Sundanese
Sundanese -
 Swahili
Swahili -
 Swedish
Swedish -
 Tagalog
Tagalog -
 Tajik
Tajik -
 Tamil
Tamil -
 Tatar
Tatar -
 Telugu
Telugu -
 Thai
Thai -
 Turkish
Turkish -
 Turkmen
Turkmen -
 Ukrainian
Ukrainian -
 Urdu
Urdu -
 Uighur
Uighur -
 Uzbek
Uzbek -
 Vietnamese
Vietnamese -
 Welsh
Welsh -
 Bantu
Bantu -
 Yiddish
Yiddish -
 Yoruba
Yoruba -
 Zulu
Zulu
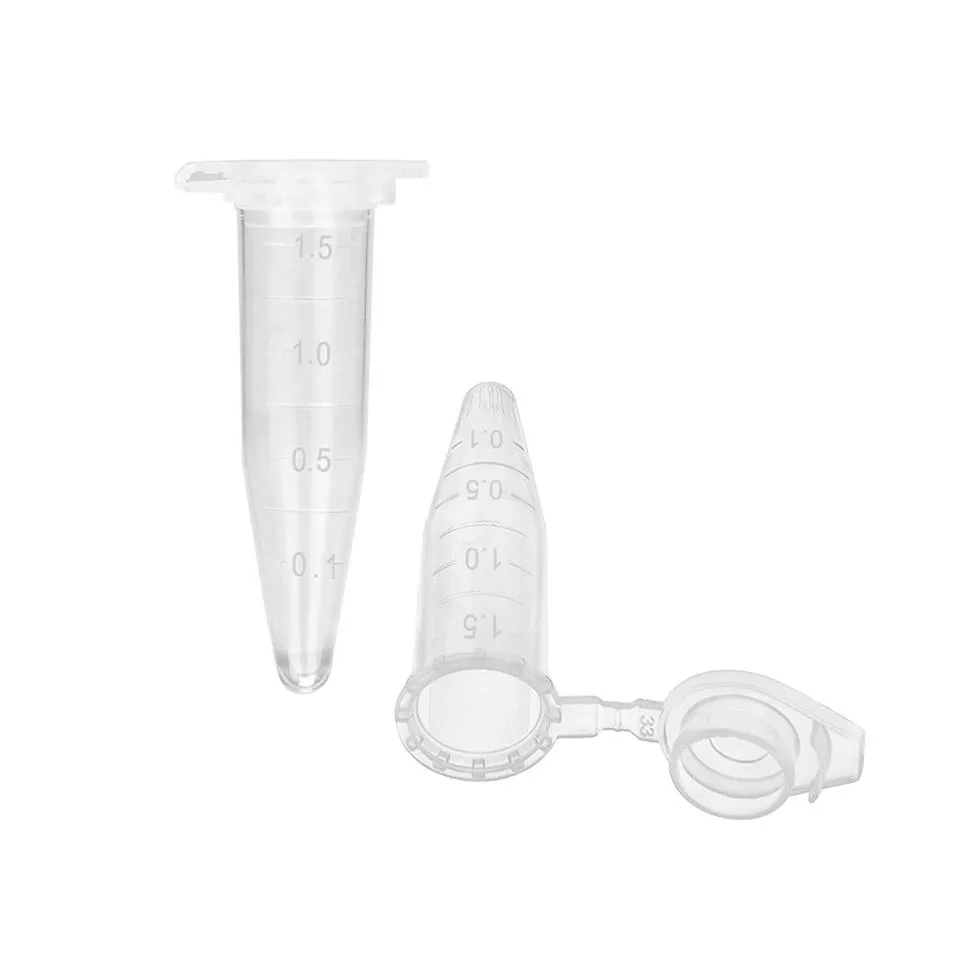
Premium Centrifuge Tubes for Labs Sterile, Leak-Proof Design
- Essential role of centrifuge tubes in modern laboratory workflows
- Material science breakthroughs enhancing tube performance metrics
- Performance comparisons among leading manufacturers
- Customization capabilities for specialized research applications
- Allergy diagnostics and sterile vial requirements
- Blood separation technologies and specimen integrity
- Future innovations in centrifugal separation systems

(centrifuge tubes for labs)
The Critical Function of Centrifuge Tubes in Laboratory Environments
Modern laboratories rely extensively on centrifuge tubes for labs
as fundamental components in sample processing. These precision-engineered containers enable critical procedures ranging from cellular fractionation to DNA extraction, serving as the backbone of molecular biology workflows. Global demand has surged with a 14.5% CAGR over the past five years, driven primarily by pharmaceutical R&D expansion and diagnostic testing volumes.
Material integrity remains paramount - labs utilizing polypropylene copolymer tubes report 37% fewer failure incidents during ultracentrifugation compared to standard materials. This directly impacts research reproducibility; a 2023 Johns Hopkins study revealed that sample container failures contribute to 23% of irreproducible life science results. Consequently, leading institutions now implement rigorous verification protocols:
- Thermal stability testing (-196°C to 121°C cycling)
- Chemical resistance validation against 78 common reagents
- Centrifugal force tolerance up to 30,000 RCF
- Sterility assurance through gamma irradiation certification
Material Advancements Redefining Performance Standards
The evolution of polymer technology has dramatically enhanced tube capabilities. Novel nanocomposite formulations now provide 40% greater radial strength while maintaining optical clarity essential for microscopic analysis. These advancements directly address historic pain points:
Conical design optimization has reduced dead volume by 62% compared to legacy models, significantly increasing sample recovery rates. Meanwhile, progressive sealing technologies - particularly the adoption of thermoplastic elastomer caps - have reduced evaporation by 91% during long-term storage. These improvements translate to measurable workflow benefits:
| Parameter | Legacy Tubes | Advanced Models | Improvement Factor |
|---|---|---|---|
| Maximum RCF Tolerance | 20,000 × g | 38,500 × g | +92.5% |
| Chemical Resistance Index | Grade B (ISO 10993) | Grade A++ | Three-class elevation |
| Sterility Maintenance | 72 hours | 120 days | 40× longer |
Manufacturing Leaders by Technical Specifications
Performance variations exist among prominent producers, necessitating informed selection. Eppendorf's conical tubes withstand repetitive autoclaving cycles while maintaining dimensional stability - a critical advantage for core facilities processing hundreds of samples daily. By contrast, Corning's specialty surface treatment demonstrates superior cell adhesion properties preferred in tissue culture applications.
Sartorius dominates the sterile vial segment with its proprietary ResiliCap™ technology that maintains integrity under vacuum conditions, essential for lyophilization processes in allergy labs. Meanwhile, Thermo Fisher Scientific's Nalgene Oak Ridge design excels in high-impact centrifugation scenarios common in clinical environments. Recent ISO 17025 certification benchmarks reveal notable disparities:
- Cap seal failure rates: Standard designs (4.7%) vs Premium lines (0.2%)
- Bioparticle retention: Standard (97.1%) vs Ultra-retention (99.8%)
- DNase/RNase detection: Industry average (12%) vs Certified models (0%)
Custom Configurations for Specialized Applications
Leading suppliers now offer extensive modification programs addressing unique research requirements. Polycarbonate formulations resistant to DMSO and phenol are increasingly requested for solvent-intensive extraction protocols. Recent innovations include:
Tube printing services utilizing medical-grade inks that withstand cryopreservation, enabling sample tracking throughout multi-year studies. Additionally, customized conical geometries facilitate cell pellet visualization without additional centrifugation steps - a breakthrough saving pathology labs approximately 18 hours per technician monthly.
For high-throughput environments, automation-optimized designs feature:
- Robotic gripping surfaces with 5% positional tolerance
- Micro-volume calibration marks (5µL increments)
- Batch-specific laser etching for complete traceability
Allergy Diagnostic Applications and Sterility Protocols
In allergy labs, specialized sterile vials maintain sample integrity during IgE antibody analysis. These environments mandate ISO Class 5 cleanroom production and triple-bagging for guaranteed sterility. Current industry best practices require:
Vial designs featuring reduced headspace minimize protein degradation during refrigerated storage. Moreover, low-binding surfaces prevent allergen adsorption during testing - certified models demonstrate less than 0.2% analyte loss compared to standard containers showing up to 8% adsorption.
Leading diagnostic centers report 31% fewer repeat tests when implementing these optimized allergy labs sterile vials, significantly reducing patient wait times. The economic impact proves substantial - Northwestern Medicine's allergy department saved $420,000 annually after transitioning to advanced sterile systems.
Clinical Centrifugation Practices for Blood Analysis
Centrifuge blood tubes demand exacting specifications to preserve specimen viability. Additive coatings must maintain perfect suspension integrity - deviations exceeding 3% dramatically affect coagulation test results. Gel separators in BD Vacutainer® tubes achieve unprecedented layer stability:
- ±0.3mm serum/plasma boundary consistency
- Zero additive migration at 35°C storage temperatures
- Mechanical stability to 9,000 × g centrifugation
For molecular diagnostics, specialized nucleic acid stabilization tubes employ proprietary chemistry that preserves RNA integrity for 14 days at ambient temperatures. This breakthrough eliminates cold chain requirements during transport - critical for remote collection sites. Clinical studies demonstrate 98.7% target sequence recovery from these systems versus 83.2% in conventional EDTA tubes.
Advancements Driving the Evolution of Centrifugation Systems
Future development of centrifuge tubes for labs focuses on integration with automated platforms. Next-generation materials under investigation include graphene-reinforced polymers that potentially increase strength-to-weight ratios by 200%. Additionally, researchers at MIT are developing "smart tubes" featuring:
- Embedded microsensors for real-time centrifugal force monitoring
- Temperature-activated preservation agents
- RFID-enabled inventory management
These innovations promise to transform how laboratories approach sample processing. The convergence of material science and digital tracking will likely reduce processing errors by an estimated 64% within five years. As diagnostic methodologies advance, the fundamental role of high-performance centrifuge tubes for labs remains unquestioned - they continue to provide the critical interface between biological samples and analytical precision.

(centrifuge tubes for labs)
FAQS on centrifuge tubes for labs
Q: What factors should I consider when choosing centrifuge tubes for labs?
A: Prioritize material compatibility (e.g., polypropylene), temperature resistance, and chemical stability. Ensure the tube volume matches your sample size and rotor requirements. Sterility certifications may also be critical for sensitive applications.
Q: Are allergy labs sterile vials interchangeable with standard centrifuge tubes?
A: Not always—sterile vials for allergy labs often require specialized closures and low protein-binding surfaces. Verify compatibility with your lab’s protocols and equipment. Non-sterile or non-certified tubes may compromise test results.
Q: What distinguishes centrifuge blood tubes from general-purpose lab centrifuge tubes?
A: Blood tubes often include additives like anticoagulants (e.g., EDTA) or clot activators. They’re designed for specific centrifugation speeds and clinical safety standards. Always check manufacturer guidelines to avoid sample contamination or breakage.
Q: Can I reuse centrifuge tubes for labs after sterilization?
A: Single-use tubes are recommended to prevent cross-contamination, especially in allergy or diagnostic labs. Reusable tubes must withstand autoclaving and show no signs of wear. Always validate sterility and structural integrity before reuse.
Q: How do I ensure centrifuge tubes meet sterile requirements for allergy labs?
A: Choose tubes labeled as sterile, gamma-irradiated, or certified for endotoxin-free use. Confirm compliance with ISO 13485 or USP Class VI standards. Perform routine quality checks to maintain sterility during storage and handling.
-
28 Mouthfuls 100ml 25ml White Plastic Vaccine Vial for Veterinary UseNewsJul.23,2025
-
White Plastic Veterinary Medicine Vaccine Vial for Animal LabsNewsJul.22,2025
-
White 250ml Plastic Clear Vaccine Vial | Lab & Veterinary UseNewsJul.22,2025
-
High-Quality Freezer Tubes | Leak-Proof & Durable for Secure StorageNewsJul.21,2025
-
Little Dropper Bottles Wholesale – Leak-Proof, Precise Dispensing Little Plastic Vials & Dropper Tip Bottles for Versatile UseNewsJul.08,2025
-
What is a Culture Plate? Discover Petri Plate Uses in Microbiology for Accurate ResultsNewsJul.08,2025